CATALIS Celebrates Four Years of Success With the FAST TRACK Evaluation Service
CATALIS is proud to celebrate four years of success for the FAST TRACK Evaluation Service, which continues to demonstrate its effectiveness in optimizing administrative processes in clinical research in Quebec.
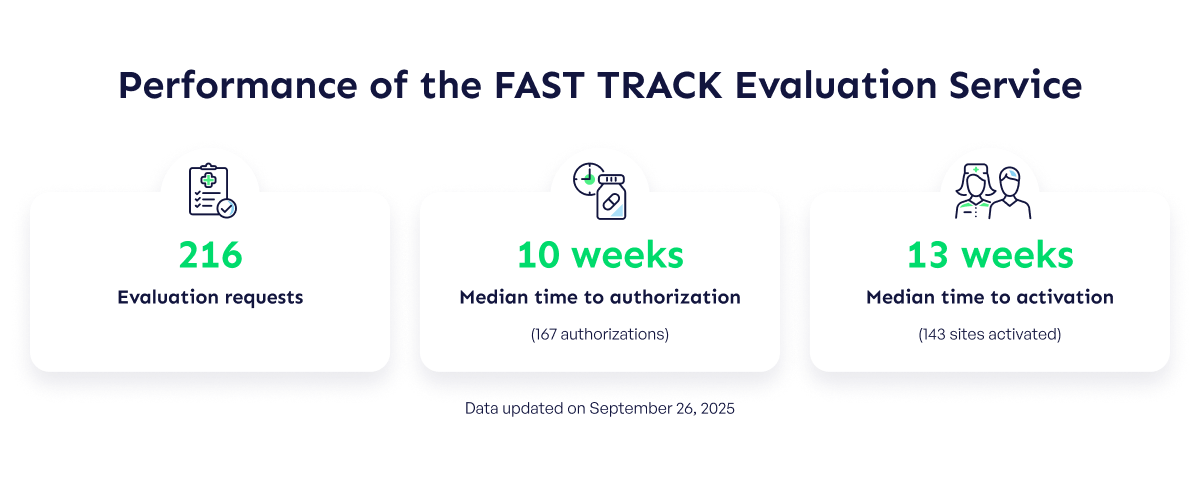
Since the launch of the pilot project in 2021, 216 evaluation requests have been processed by the CATALIS Central Office and then evaluated by participating healthcare institutions. To date, and thanks to the collective efforts of all stakeholders, the Service’s performance is ~70% faster than typical median times.
- Scientific, ethical, and contractual approvals are obtained in a median time of 7 weeks.
- Institutional authorizations are obtained in a median time of 10 weeks, which includes all project evaluations: scientific, ethical, contractual, pharmacy, imaging, laboratory, and other required local conveniences.
- Site activation is completed in 13 weeks, which corresponds to the total median time elapsed between receipt of study materials for evaluation and site activation by the sponsor.
In 2024-2025, the FAST TRACK Evaluation Service processed 11% of pharmaceutical clinical trials submitted in Quebec, and our projections indicate that this volume will double in 2025-2026.
An Effective Ethical Review Process
While several pan-Canadian stakeholders express concerns about the cumbersome nature of ethical evaluation processes, Quebec stands out for the remarkable efficiency of its research ethics boards (REBs) within the service framework.
As early as 2021, public-private members of the CATALIS Network reviewed processes to ensure the quality of project submissions and optimize exchanges with REBs. Combined with the provincial multicenter ethics mechanism, these adjustments, made both upstream and downstream of ethical evaluation, have significantly reduced REB approval times.
This performance is all the more notable as REB evaluations in Quebec cover several dimensions: scientific evaluation, overall project ethics, as well as local ethics related to its implementation. They include verification of recruitment methods, consent processes, conflicts of interest, compliance with provincial laws and regulations, translation of materials for participants, administrative modifications to consent forms by sites, and consideration of institutional specificities.
Through this rigorous and collaborative approach, the FAST TRACK Evaluation Service has demonstrated over four years its ability to concretely reduce clinical trial startup timelines, thus enabling healthcare institutions and sponsors to accelerate access to innovation, and allowing patients faster access to clinical trials in Quebec.
If you would like more information about the FAST TRACK Evaluation Service, please write to us at: info@catalisquebec.com.