Launch of Clinical Trials Quebec, Powered by Catalis, in Partnership With the Quebec Government
Clinical trials are an essential step in the successful development of any innovative treatment that benefits our society. Yet few people understand their importance. This is why CATALIS Quebec is proud to have coordinated the development of Clinical Trials Quebec (CTQ), a brand-new information and services hub for all things clinical research.
CTQ is the culmination of a unique, collaborative effort by the CATALIS Network. We would like to thank the ministère de la Santé et des Services sociaux du Québec (MSSS), the ministère de l’Économie, de l’Innovation et de l’Énergie (MEIE), Health Canada, the Institut national d’excellence en santé et en services sociaux (INESSS), the institutions of the Quebec healthcare network, patient associations and patient partners, and every other member of the CATALIS Network, who contributed to creating this groundbreaking provincial tool.
Clinical Trials Quebec: A New Provincial Resource on Clinical Research
Clinical Trials Quebec’s (CTQ) website offers impartial, comprehensive, and accessible information on clinical research, as well as tools and services to improve Quebecers’ access to clinical trials. This initiative is consistent with CATALIS’ mission: to optimize the clinical research environment in Quebec in order to accelerate the development of innovative patient care.
Learn More About the CTQ Initiative
Engaging Educational Content for the General Public and Patients
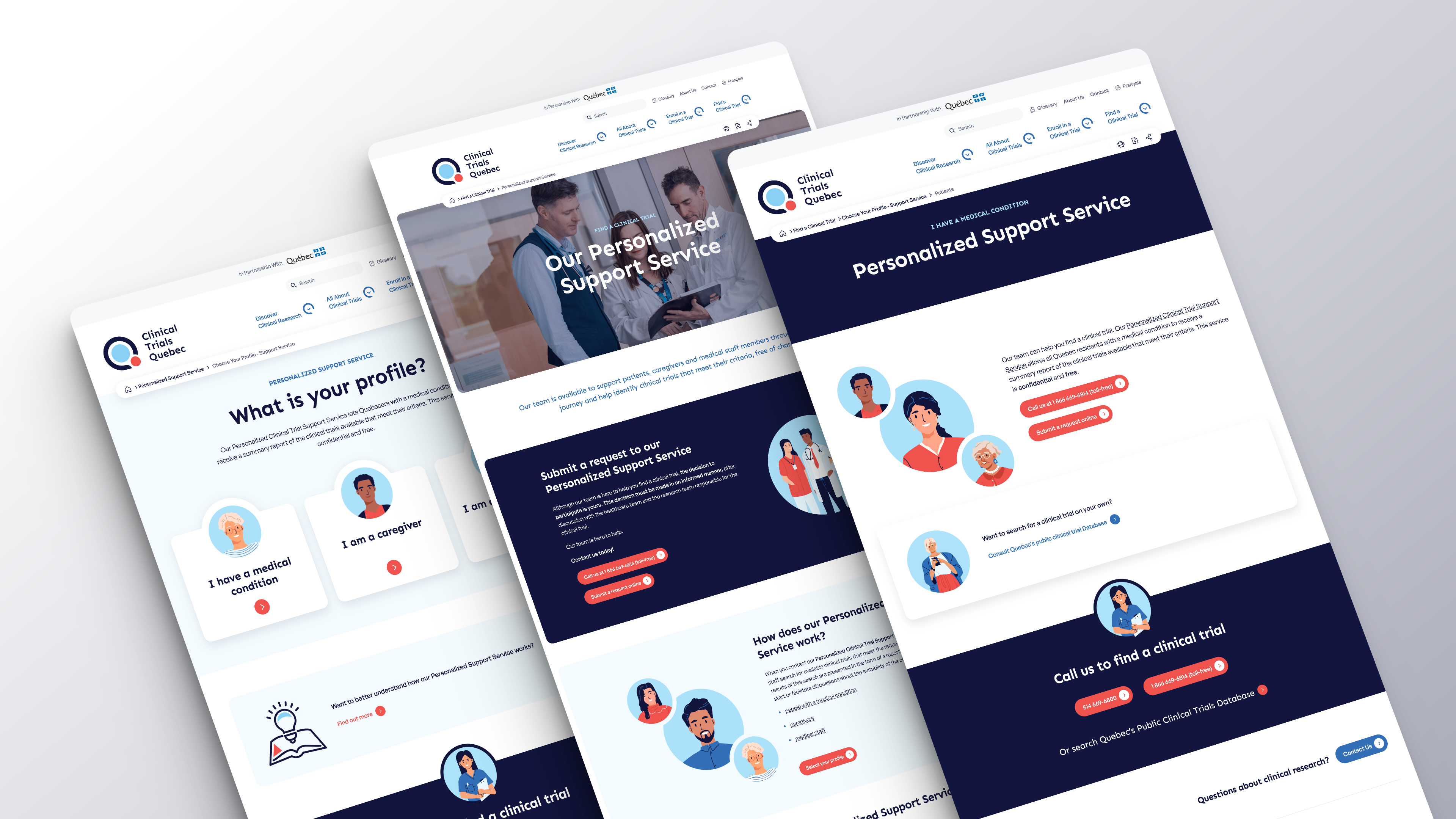
Clinical Trials Quebec (CTQ) provides Quebecers with a comprehensive and accessible overview of clinical research, from its basic definition to more in-depth subjects such as enrolment processes and regulatory frameworks. Infographics and online tools, such as a Directory of Patient Organizations and Quebec’s Public Clinical Trials Database, allow everyone to obtain information on clinical research. In addition, a Personalized Support Service helps patients, caregivers and medical staff identify clinical trials that match their criteria.
Discover the Importance of Clinical Research
Intuitive Tools for Understanding the Clinical Trials Regulatory Process
The clinical research regularory process is essential to guarantee participants’ safety and the validity of results. The regulatory process also serves to ensure that new medical treatments and medical advances meet the highest standards of ethics and quality. CTQ has worked with Health Canada and the Institut national d’excellence en santé et en services sociaux (INESSS) to develop materials explaining the main steps involved in developing a new drug. To help everyone understand the content of the website, CTQ also offers a glossary of complex medical terms.
View the Clinical Trials Regulatory Process
Quickly Find a Clinical Trial Thanks to our Personalized Support Service!
Clinical Trials Quebec’s (CTQ) Personalized Support Service staff can conduct a thorough search of available clinical trials that meet specific criteria, free of charge. The search results are then quickly submitted in the form of a report, enabling people with a medical condition, caregivers, and medical staff to discuss the suitability of enrolling in a clinical trial.
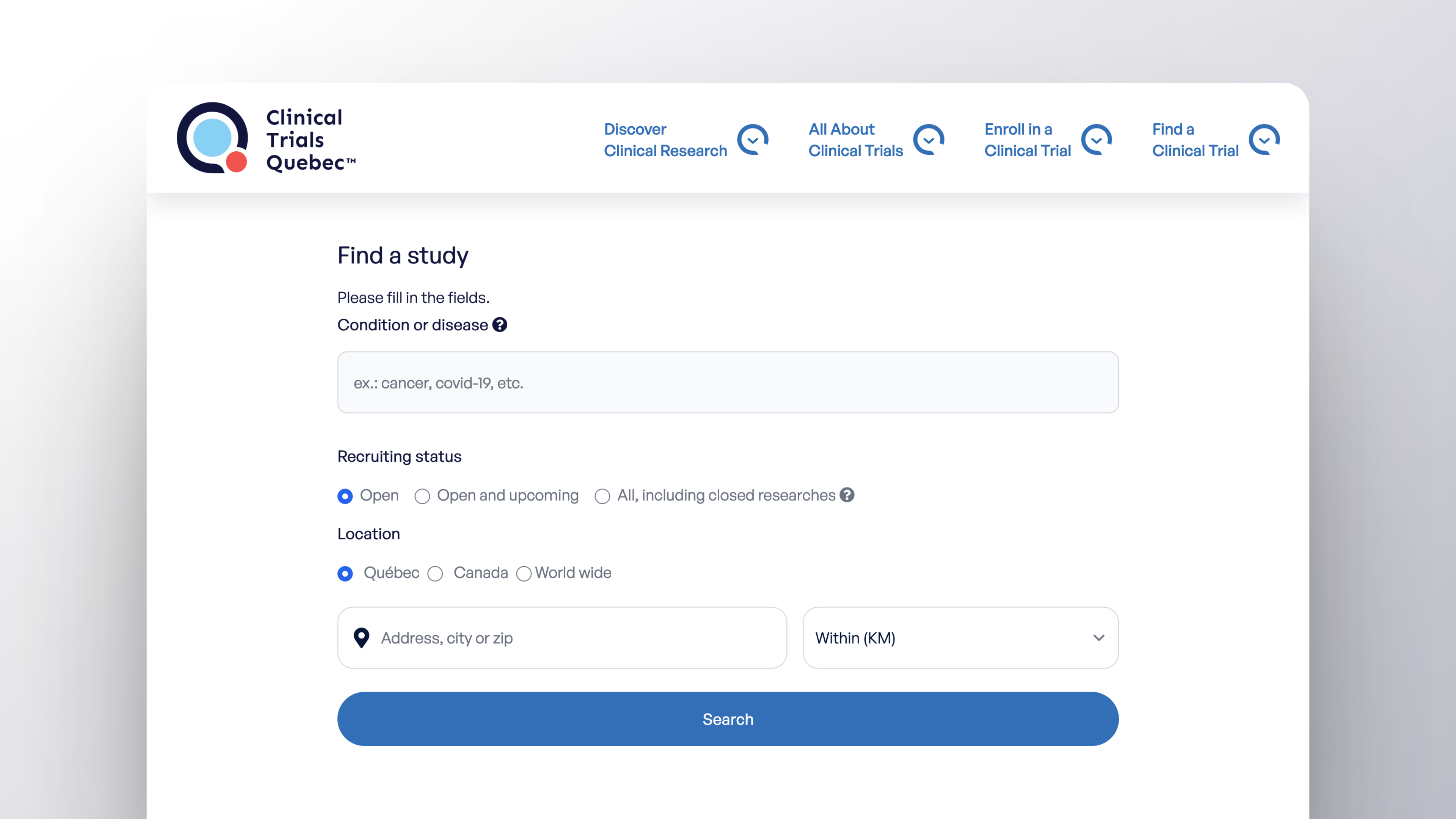
Look for Clinical Trials Using Quebec’s Public Clinical Trials Database!
Easily find clinical trials being conducted in Quebec, in Canada, and around the world. Quebec’s Public Clinical Trials Database is available on CTQ’s website. It was developed by SemiWeb using the Nagano platform. This provincial Registry automatically aggregates and shares data in real time from Quebec’s public health and social services institutions, as well as data from ClinicalTrials.gov (an international public database).
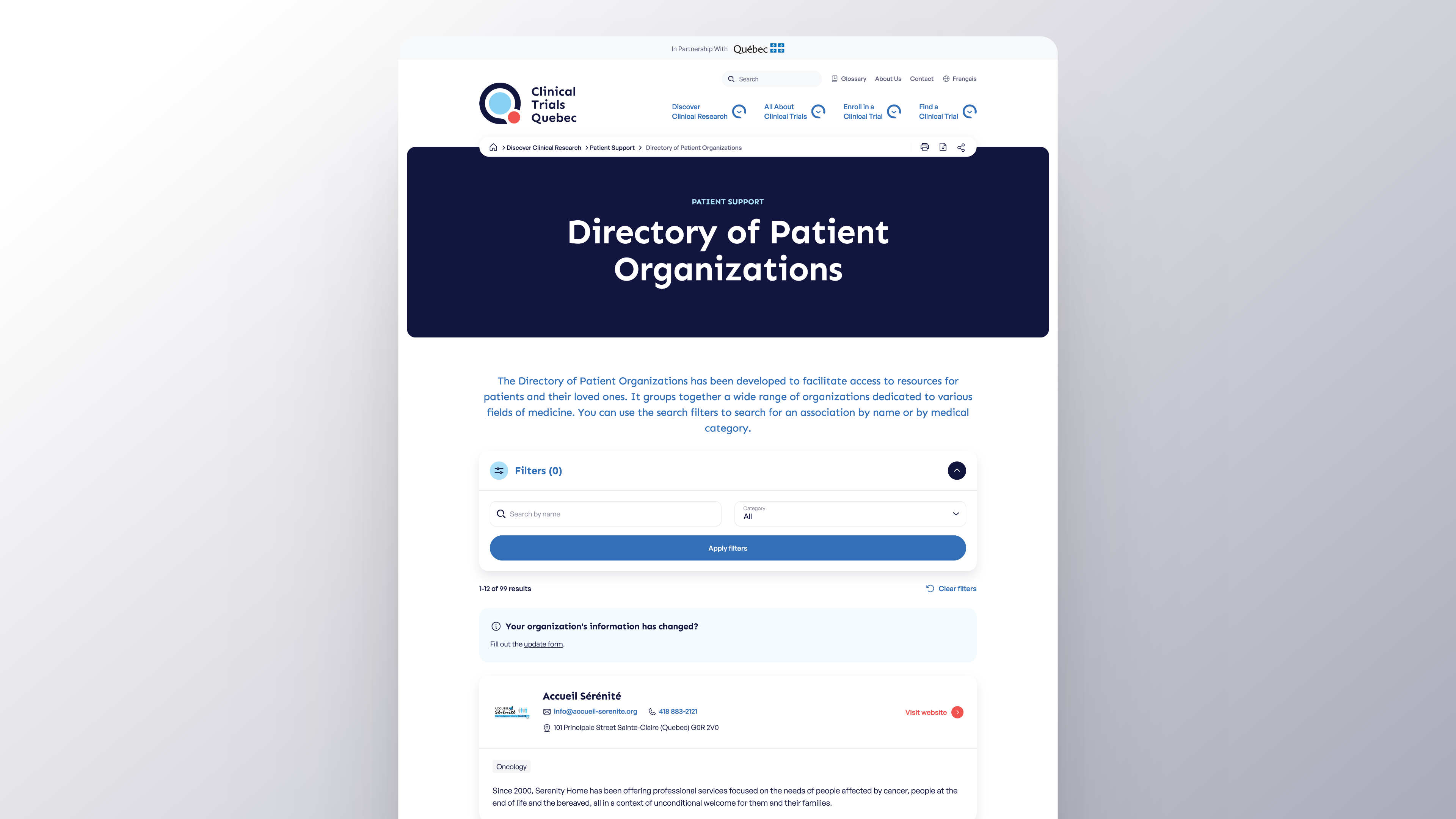
Find a Patient Organization in Our Directory!
Find a wide range of patient organizations in Quebec, covering a variety of medical fields using CTQ’s Directory. Patient organizations play an essential role in Quebec’s healthcare system by representing patients, facilitating access to better care, and supporting clinical research.
Do you have questions about clinical research or the CTQ information hub? Would you like to share your opinion with us? Selecting the subject of your request via our contact form and submitting it is easy as a breeze. Our team will be happy to answer you as soon as possible.
See you soon on the Clinical Trials Quebec Website!